The AI Vision System for Ensuring Quality and Traceability in Life Sciences
Automated inspection that ensures compliance, protects product integrity, and maintains full traceability from assembly to packaging.
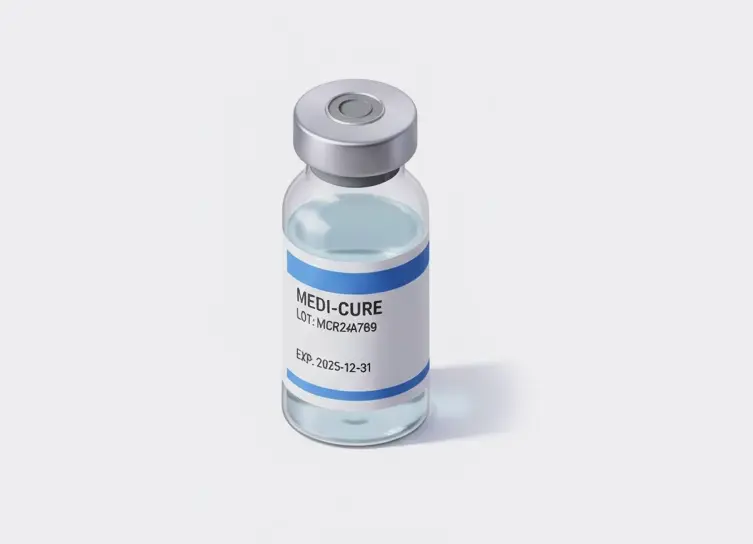
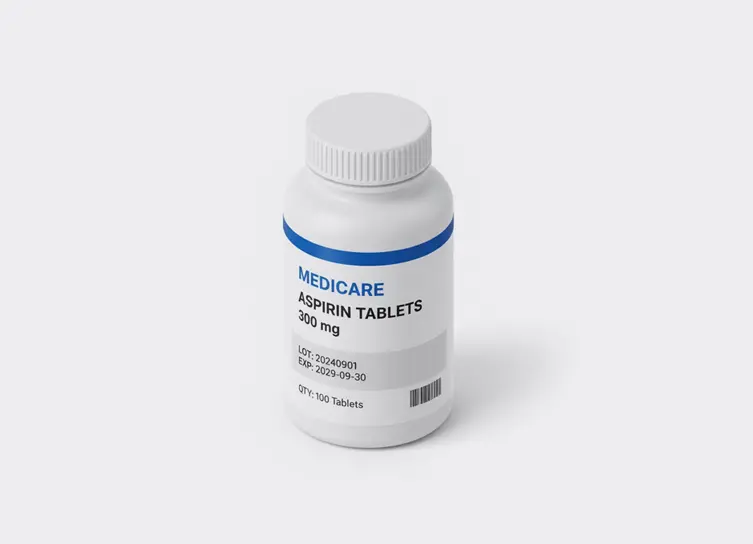
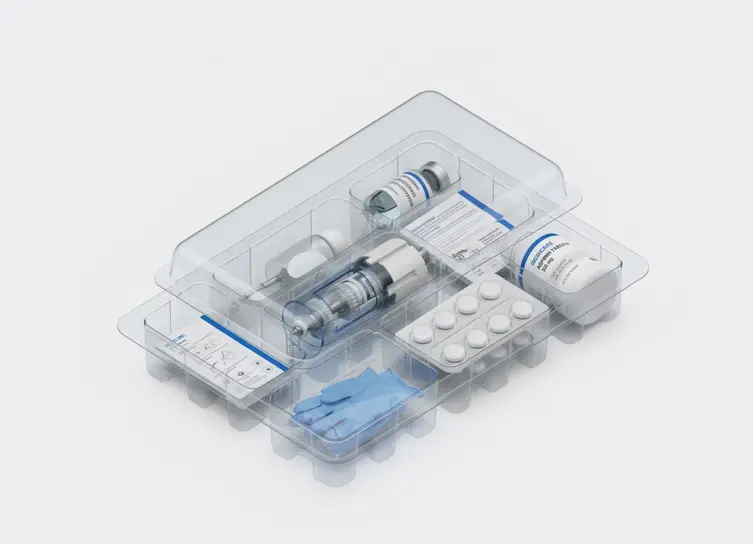
In life sciences manufacturing, precision and compliance are critical at every step. From medical device assembly to pharmaceutical packaging, even small errors—like a missing lot code, a cracked vial, or a misaligned stopper—can lead to recalls, regulatory violations, or patient risk. Automated vision inspection helps manufacturers achieve zero-defect quality, complete traceability, and audit-ready documentation across their production lines.
Key
Applications
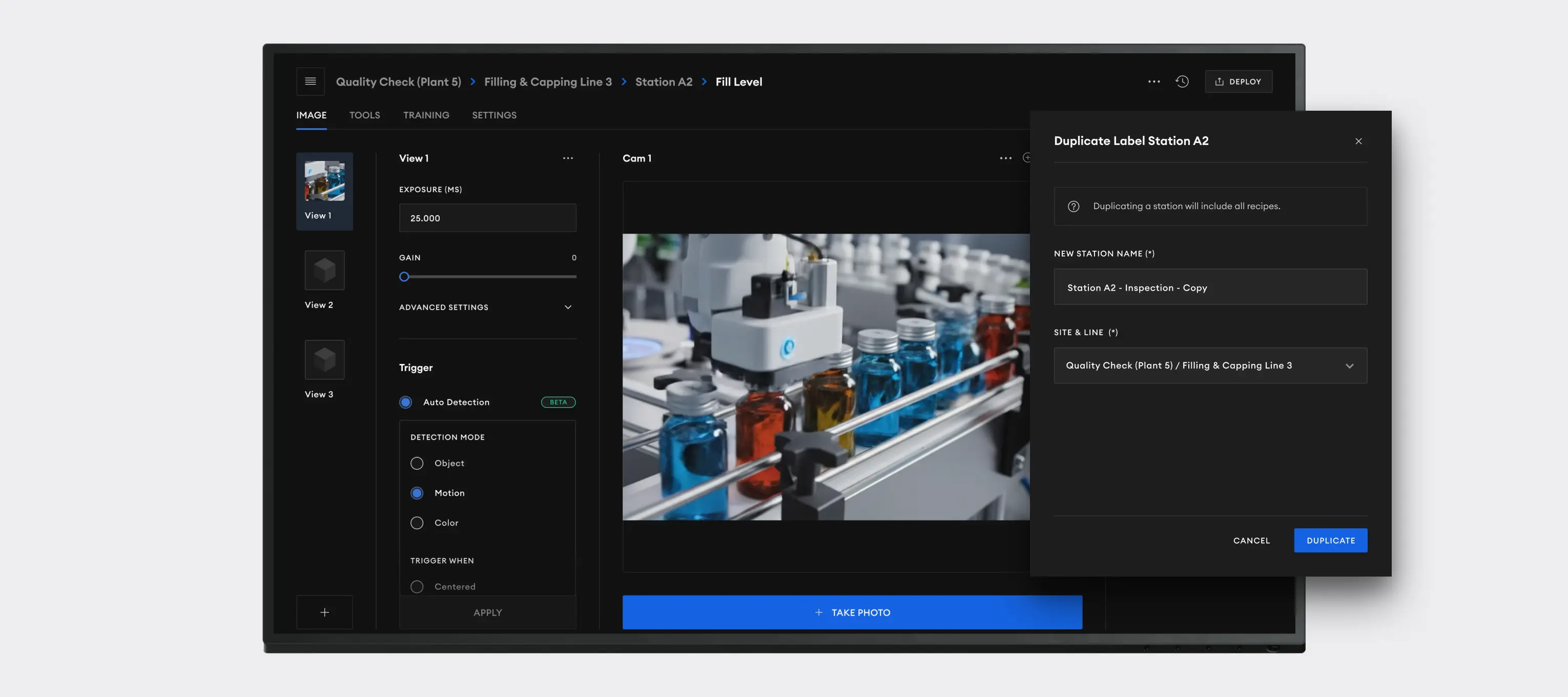
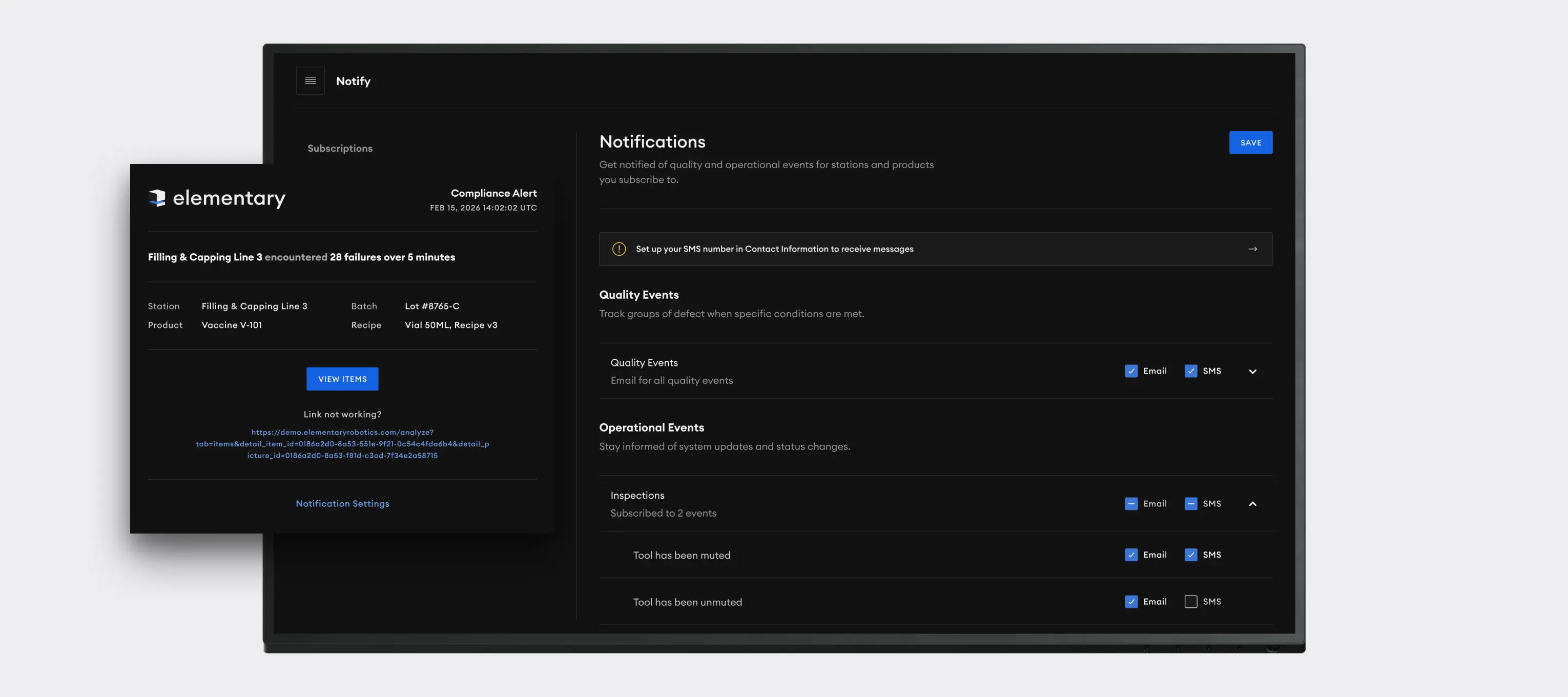
Drive zero-defect quality and compliance by using AI machine vision to perform microscopic defect detection, component verification, and comprehensive track-and-trace monitoring across all critical manufacturing stages, ensuring patient safety and regulatory adherence.
Key Features
and Benefits
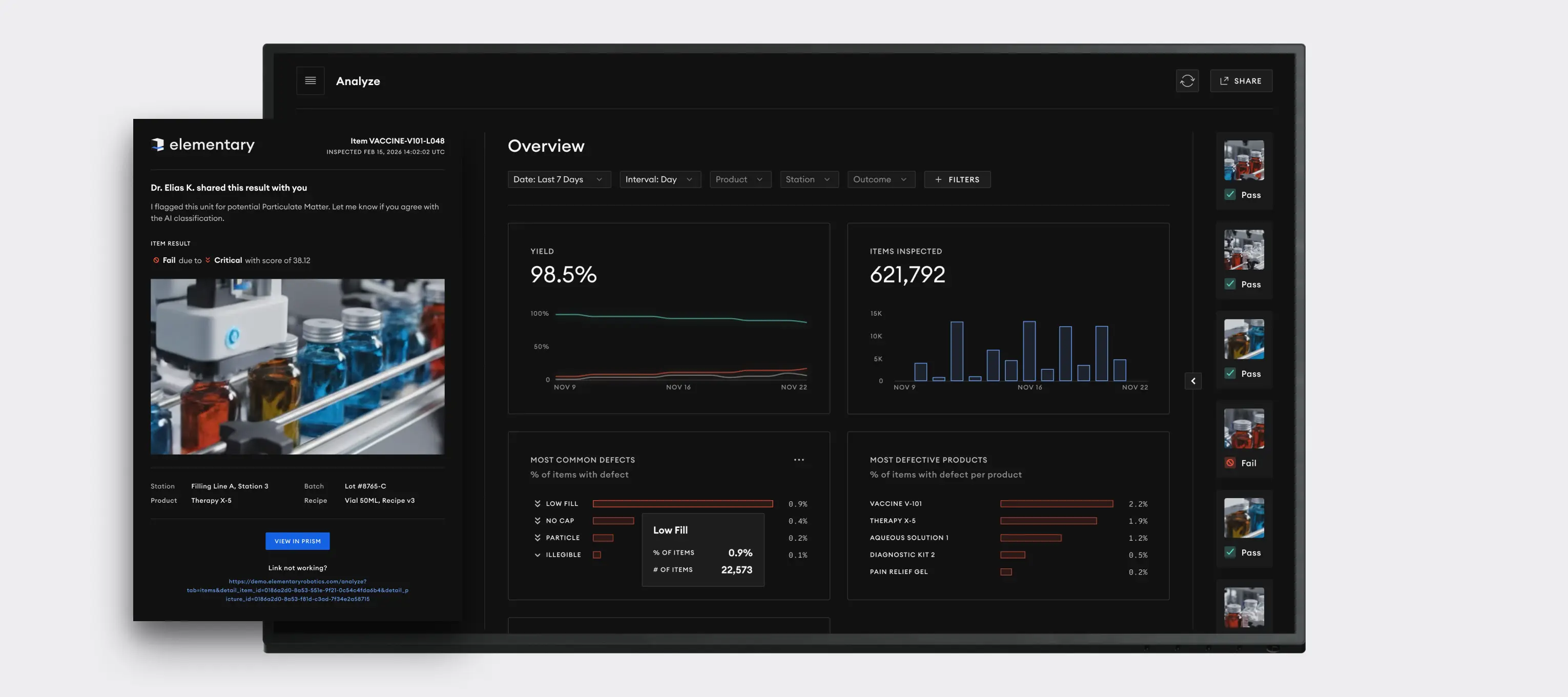
Elementary's AI-driven vision system provides a strategic advantage by transforming complex inspection data into clear, actionable insights, enabling robust regulatory compliance, maximizing product yield, and ensuring brand protection through verifiable quality.